Introduction: sASCT in multiple myeloma (MM) induces superior durability of response over non-transplant consolidation 1. Currently there is no prospective evidence for post-sASCT consolidation and maintenance. This is important given that most patients relapsing after first ASCT (ASCT1) are lenalidomide refractory (Len-Ref) following maintenance. The role of a proteasome inhibitor (PI) containing post-sASCT consolidation and maintenance strategy is explored in UK-MRA Myeloma XII (ACCoRD) trial using the second-generation oral PI, ixazomib. The results of a planned interim analysis (IA) of efficacy and safety are presented here.
Patients and Methods: ACCoRD enrolled patients who relapsed, requiring treatment more than 12m after ASCT1, delivering an oral PI/IMID re-induction regimen (ixazomib, thalidomide and dexamethasone; ITD) prior to randomization between sASCT conditioned with high-dose melphalan (HDM) vs. ixazomib-augmented ( iMel). The second randomization between observation (standard of care in sASCT; OBS) and post-transplant ITD consolidation and ixazomib maintenance (CON/MAINT), was conducted at D100 post-sASCT. The primary endpoint was progression-free survival (PFS). Responses were assessed in accordance with IMWG criteria with MRD defined by the limit of the multi-parameter flow assay (<10 -5). All patients underwent genetic risk categorization by a central laboratory with high-risk (HiR) classified as presence of t(4;14), t(14;16), t(14;20), del(17p) or gain(1q) and ultra-high risk (UHiR) the presence of more than one lesion. Pre-planned subgroup analysis included: genetic risk status (SR/HiR/UHiR) and previous treatment received (PI exposure/Len-Ref).
Results: ACCoRD recruited between March 2017 and June 2022, with database lock for IA in May 2023. 496 patients were enrolled and 206 patients randomized in this comparison (103 CON/MAINT, 103 OBS). Median age was 62.5y (range 34, 78) with 35.7% patients >65y. The median observed TTP from ASCT1 was 32m (range 2, 212), with 12.5% patients relapsing after lenalidomide maintenance (Len-Ref). The proportion of patients with an ASCT1 TTP <18m, 18-24m and >24m was 13.5%, 15.1% and 71.5%, respectively. Of those with complete genetic results at trial entry, 58.3% had standard risk, 31.1% had HiR and 10.7% had UHiR disease. 61.5% of patients were PI-exposed. The median time from sASCT to CON/MAINT was 4.3m (3.4, 6.7). The ORR following sASCT was 83.3%, with ³VGPR in 50.5% and MRD negative in 19.5%.
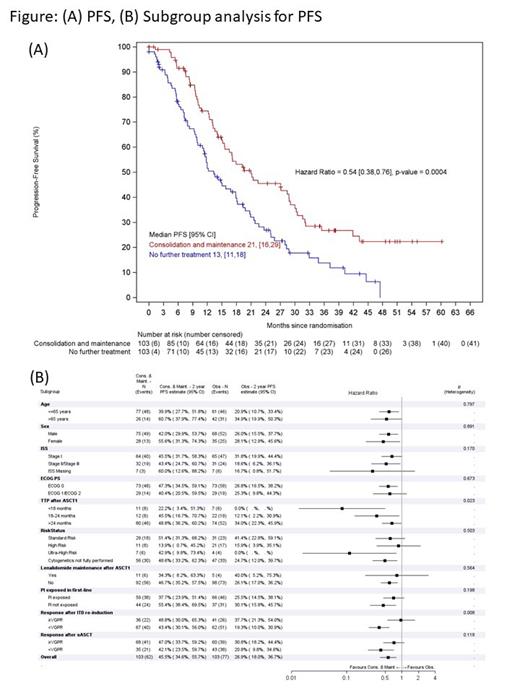
After median follow-up of 27.3m (IQR 13.1, 38.4), 139 patients had progressed or died (CON/MAINT 62, OBS 77). ITT analysis showed CON/MAINT was associated with longer PFS than OBS (HR 0.54, 95% CI 0.38, 0.76, median PFS CON/MAINT 21m vs. OBS 13m, p=0.0004, Figure 1A).
Subgroup analyses (Figure 1B) suggested that there were similar benefits for PFS in terms of baseline characteristics: age, sex, ISS and performance status. There was similar benefit for patients by previous treatment received (PI/Len). However, patients with the shortest remission after ASCT1 achieved the greatest benefit with CON/MAINT (p=0.023). Patients achieving poor response (<VGPR) after ITD re-induction benefitted most from CON/MAINT (P=0.008).
45 patients had a PFS2 event (18 CON/MAINT, 27 OBS). CON/MAINT showed a non-significant improvement with longer PFS2 than OBS (HR 0.56, 95%CI 0.30, 1.01, median PFS2 CON/MAINT NR vs. OBS 44m, p=0.055). 25 patients have died (9 CON/MAINT, 16 OBS). Similarly, CON/MAINT showed a non-significant improvement with longer overall survival (OS) than OBS (HR 0.48, 95%CI 0.21, 1.09, 3y OS: CON/MAINT 81.9% vs. OBS 70.4%). Interpretation of PFS2 and OS are limited by short follow-up and will be updated at final analysis.
CON/MAINT was well tolerated with no significant additional toxicity. 87.4% of patients on the CON/MAINT arm completed CON treatment. For those who completed CON, MAINT was administered for median 15 cycles (range 1, 57). The most common grade 3+ adverse events in CON/MAINT were upper respiratory infection (6.8% of patients) thrombocytopenia (5.8%), lung infections (2.9%), lymphopenia (2.9%) and neutropenia (2.9%).
Conclusion: This IA demonstrates a clear advantage in durability of response when sASCT is followed by consolidation and maintenance which is consistent across subgroups. The impact on PFS2, OS and quality of life remains to be clarified.
1.Cook G, et al The Lancet Oncology 15(8), 874-885.
OffLabel Disclosure:
Cook:Amgen: Consultancy; Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharma: Consultancy. Ashcroft:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hockaday:Abbvie: Speakers Bureau. Snowden:Kiadis Pharma: Membership on an entity's Board of Directors or advisory committees; Sanofi: Speakers Bureau; advisory boards for MEDAC and Vertex, and clinical trial IDMC membership for Kiadis: Speakers Bureau; Mallinckrodt: Speakers Bureau; Advisory boards for Vertex: Speakers Bureau; Jazz: Speakers Bureau; Janssen: Speakers Bureau. Garg:Alnylam: Honoraria; Celgene: Other: Ad board; Stemline therapeutics Janssen: Other: Ad board; Janssen: Consultancy, Honoraria, Other: Ad board, research support, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Ad board, travel support; Sanofi: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Ad board, travel support; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Boyd:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Speakers Bureau; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Other: Travel support. Cook:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Cairns:Celgene BMS: Honoraria, Research Funding; Sanofi: Research Funding; Amgen: Research Funding; Janssen: Honoraria; Takeda: Research Funding. Parrish:BMS Celgene: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Everything Genetic: Consultancy; Janssen: Speakers Bureau; Jazz: Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria.
Ixazomib is used in combination with thalidomide and dexamethasone as re-induction and consolidation around salvage autologous stem cell transplantation (sASCT). In addition, ixazomib is used as augmented conditioning for sASCT and maintenance therapy post-sASCT.